
PPT Solutions PowerPoint Presentation, free download ID6751365
A supersaturated solution is a homogeneous mixture in which the solute is not in equilibrium with its undissolved form. This is equivalent to saying that a supersaturated solution has a.

PPT Solutions Unit PowerPoint Presentation, free download ID1980079
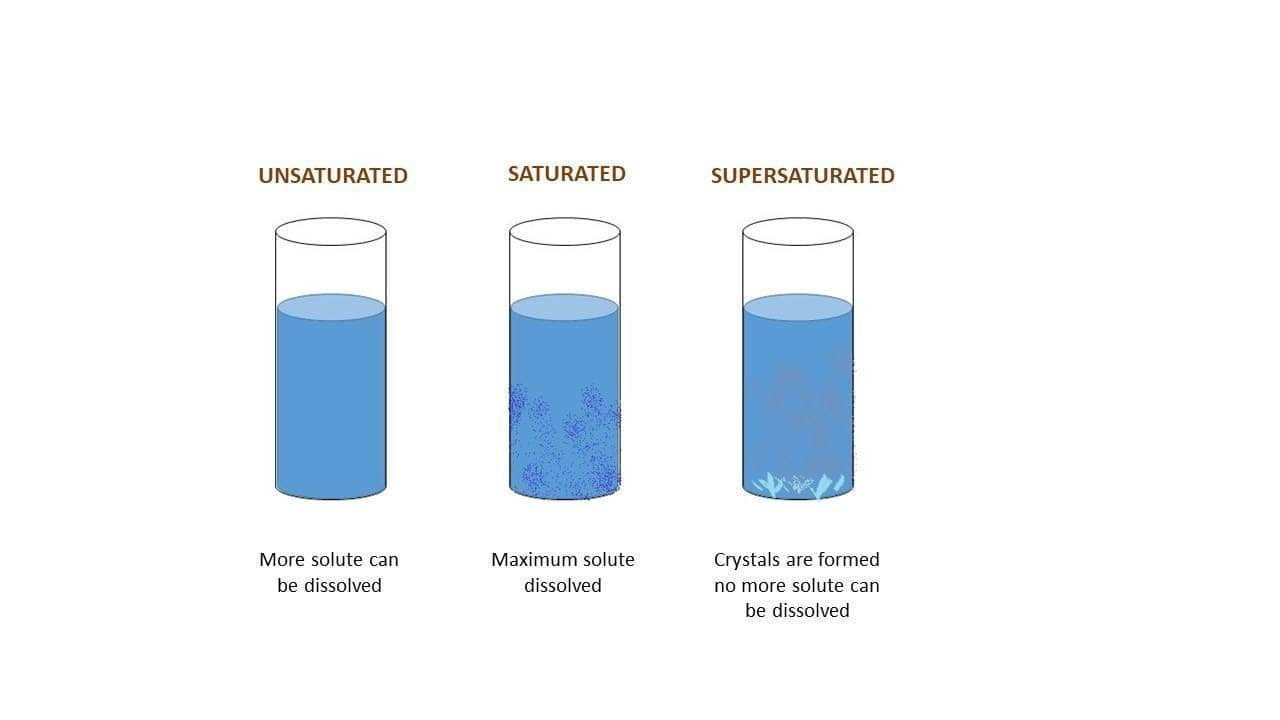
A saturated solution is a chemical solution containing the maximum concentration of a solute dissolved in the solvent. The additional solute will not dissolve in a saturated solution. The amount of solute that can be dissolved in a solvent to form a saturated solution depends on a variety of factors. The most important factors are:
STOCK IMAGE, example of a supersaturated solution one that contains moreof a solute than can be
This type of solution is metastable. The excess dissolved solute is recrystallized by adding a tiny crystal of solute called seed crystal. An example of a supersaturated solution is carbon in soft drinks. Due to pressure, more than the maximum carbon is dissolved into the solvent. Also, the sugars in clear honey are supersaturated.
supersaturated solution Overview, Structure, Properties & Uses
A supersaturated solution contains more solute at a given temperature than is needed to form a saturated solution. Increased temperature usually increases the solubility of solids in liquids. For example, the solubility of glucose at 25 °C is 91 g/100 mL of water. The solubility at 50 °C is 244 g/100 mL of water. If we add 100 g of glucose to 100 mL water at 25 °C, 91 g dissolve.

PPT Chapter 13 Properties of Solutions PowerPoint Presentation, free download ID480288
Home Under Construction Purgatory Types of Saturation Types of Saturation Page ID Introduction When solid solute ( substance or particles) and liquid solvent are mixed, the only possible reactions are dissolution and crystallization. Dissolution is the dissolving process of the solid solute.

Supersaturated Solution Definition and Examples
A supersaturated solution is in a metastable state; it may return to equilibrium by separation of the excess of solute from the solution, by dilution of the solution by adding solvent, or by increasing the solubility of the solute in the solvent. History Solubility of Na 2 SO 4 in water as a function of temperature.

Precipitation of a Supersaturated Solution YouTube
Solution. Verified by Toppr. Supersaturated solution: A solution that contains more than the maximum amount of dissolved solute than a saturated solution under the same conditions and no more solute can be added to the solution until the temperature is raised. Hence, the correct option is A.

PPT Chapter 4 PowerPoint Presentation, free download ID4638445
supersaturated solution: A solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature.

PPT Topic 7 Solutions PowerPoint Presentation, free download ID5738045
Chemistry Physical Chemistry Saturated Unsaturated and Supersaturated Saturated Unsaturated and Supersaturated Honey is a fascinating solution, made up of approximately 70% sugar and 20% water. Honey contains more sugars than what would normally dissolve at room temperature, and it is considered a supersaturated solution!

PPT Solutions PowerPoint Presentation, free download ID269800
A supersaturated solution definition or supersaturated solution definition chemistry is that as the solution containing more than the maximum amount of solute that is able to dissolve in solvent at a particular given temperature. A supersaturated solution possesses an unstable state; it could be made stable by separating the excess amount of.

PPT Solutions PowerPoint Presentation, free download ID765271
A heat pack contains a supersaturated solution of material such as sodium acetate. The solution is clear until a small metal trigger is activated. The sodium acetate then crystallizes out of solution and generates heat in the process. Figure 16.5.1 16.5. 1: A thermal pack is applied to heat or cool muscles.

Supersaturated Solution Definition Chemistry DEFINITIONHD
10.16: Saturated and Supersaturated Solutions. We often find that there is a limit to the quantity of solute which will dissolve in a given quantity of solvent. This is especially true when solids dissolve in liquids. For example, if 36 g KCl crystals is shaken with 100 g H 2 O at 25°C only 35.5 g of the solid dissolves.

PPT Solutions PowerPoint Presentation, free download ID6594558
Supersaturation. Supersaturated Solutions. Increased temperature usually increases the solubility of solids in liquids. To understand why, we need to return to the Second Law of Thermodynamics. Increased temperature means a greater average velocity for the particles. This allows them to move from one position to another more easily.

PPT Solutions Vocabulary PowerPoint Presentation, free download ID9652529
A supersaturated solution contains more dissolved solute than required for preparing a saturated solution and can be prepared by heating a saturated solution, adding more solute, and then cooling it gently. Excess dissolved solute crystallizes by seeding supersaturated solution with a few crystals of the solute. Table of Contents Recommended Videos

PPT Solutions PowerPoint Presentation, free download ID2965441
By definition, a supersaturated solution is a chemical solution that contains more solute than the solvent can hold. In other words, a supersaturated solution has more dissolved solute than a saturated solution. The process of forming a supersaturated solution is called supersaturation.

PPT Solutions PowerPoint Presentation, free download ID2869731
A Supersaturated Solution definition is given as the one, which contains more dissolved solute than needed for preparing a saturated solution and is prepared by heating a saturated solution, adding excess solute, and then by gently cooling it.
